NIH Other Support
Information on NIH Other Support.
Background
In light of events affecting the “integrity of biomedical research” in US research over the past few years, NIH issued NOT-OD-19-114. The intent of this Notice was not to introduce the research extramural community to new NIH policies, but instead to remind us of NIH's longstanding policies on Other Support, Financial Conflict of Interest (FCOI), and Foreign Components in the hopes of improving reporting of ALL research support (both domestic and foreign) while promoting openness and transparency.
On March 12, 2021, NIH released NOT-OD-21-073: Upcoming Changes to the Biographical Sketch and Other Support Format Page for Due Dates on or after May 25, 2021, and on April 28, 2021, NIH released NOT-OD-21-110: Implementation of Changes to the Biographical Sketch and Other Support Format Page. These two notices informed the research community of NIH's upcoming changes to both the Biosketch and Other Support. Use of the new forms was requested for applications, progress reports, JIT, post-submission/award that were due on or after May 25, 2021, and was required for applications, progress reports, JIT, post-submission/award due on or after January 25, 2022.
Training Sessions
On March 30, 2021, a Quarterly Contacts Meeting was held where upcoming changes were discussed. Since then, NIH released the above clarification notice that this new form was requested to be used for deadlines on or after May 25, 2021, and that it firmly is required for deadlines on or after January 25, 2022. Additionally, on November 10, 2021, and January 12, 2022, the topic was presented for the campus-wide Research Compliance Hot Topics and Training Program on the upcoming changes. A copy of the presentations and recordings can be found below:
- Quarterly Contracts Meeting (March 30, 2021) PPT
- Research Compliance Hot Topics Biosketch Changes November 2021 PPT
- Research Compliance Hot Topics Biosketch Changes November 2021 Recording
- Research Compliance Hot Topics Biosketch Changes January 2022 PPT
- Research Compliance Hot Topics Biosketch Changes January 2022 Recording
Reminder: Why Other Support Reviewed?
- All resources, domestic or foreign, directly supporting the individual’s research endeavors have been reported
- Sufficient levels of effort are committed to the project
- There is no scientific, budgetary, or commitment overlap
- Only funds necessary to the approved project are included in the award (examples are subawards or multi-project awards)
- Any foreign resources that meet the definition of a foreign component have received appropriate prior approval
Other Support Resources
- Other Support Form Page Overview
- Other Support Form Pages: New Other Support Form Page
- Instructions: New Other Support Form Page
- Other Support, Foreign Components, and FCOI FAQs
- NIH Pre-award and Post-award Disclosures Relating to the Biographical Sketch and Other Support (updated 6/8/2022).pdf
- Examples of What to Disclose to NIH about Senior/Key Personnel on Applications and Awards Table
- How to Flatten a PDF
Electronic Digital Signature Options
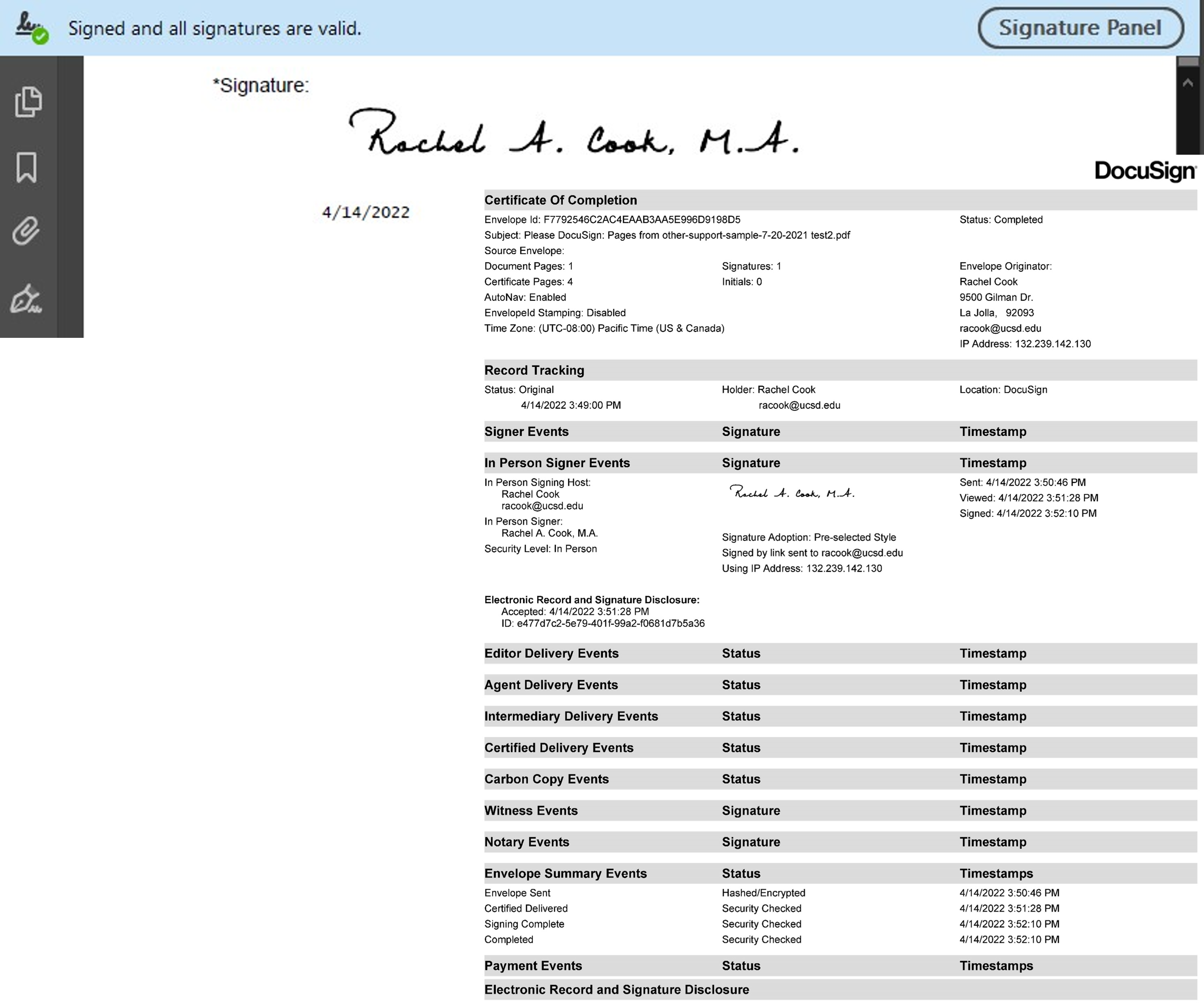
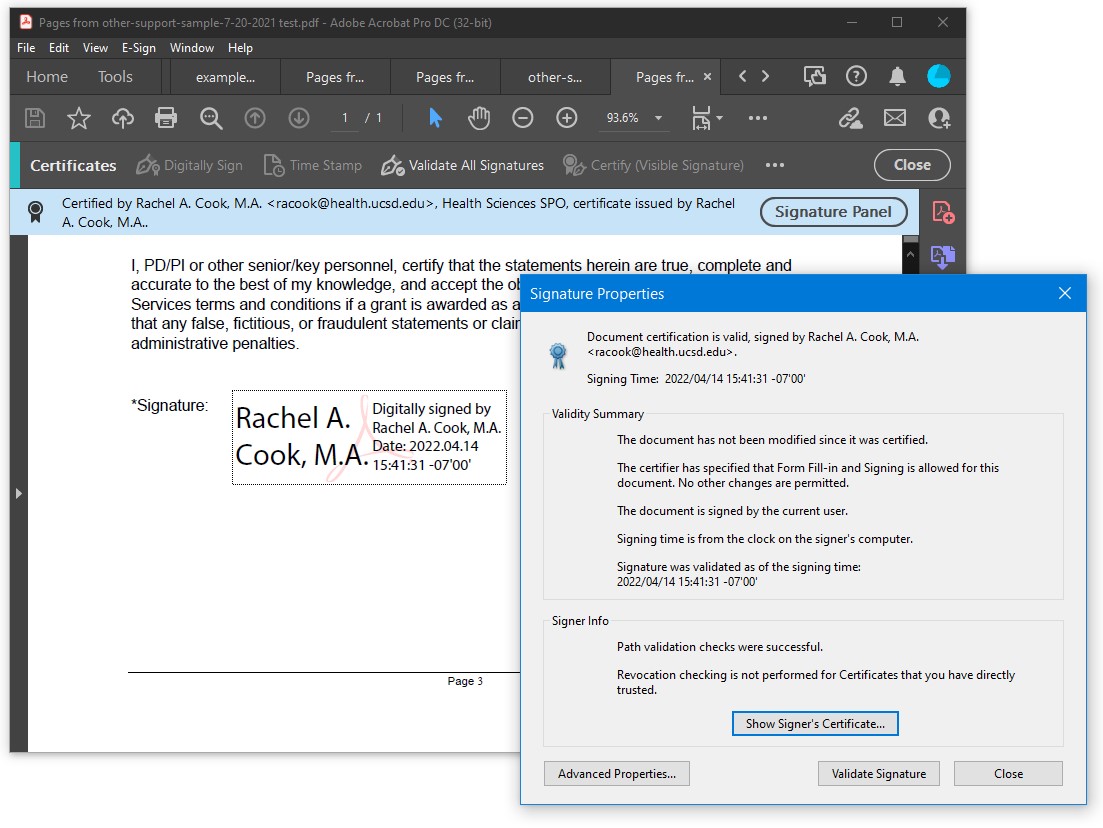
DocuSign at UC San Diego For DocuSign, you have to log in through SSO. Once there, it allows you to drag and drop the document you need to have signed. You can be both the host and signer. You can also be the host and forward an email to the person that needs to sign. For each step in the process, you will receive an email telling you it has to be signed as well as that it is complete. Once you download the document to your desktop, it will show that it has been signed and all the signatures are valid in Adobe Acrobat. When you download the document you will also download the Certificate of Completion which you must save for audit purposes. Please do not upload this to eRA Commons or ASSIST to be sent to NIH. Here is an example of the e-Signature and the Certificate of Completion.
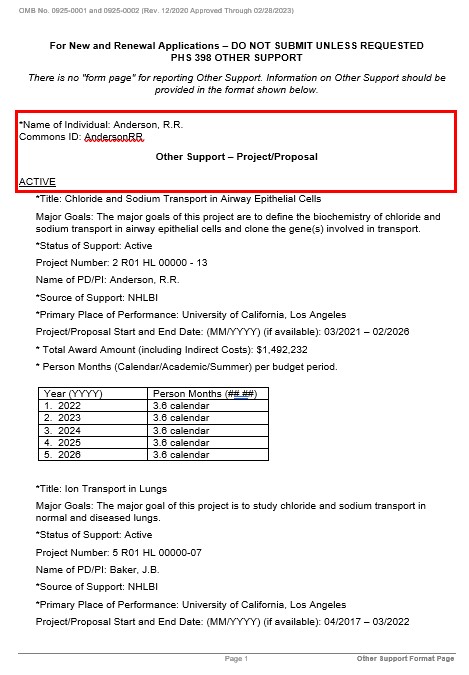
Other Support Format Detailed Information, Including a Walk-Through
Do not include past budget period efforts. Only list current and future budget period efforts. If there are extra rows in the table that are not utilized, then delete those rows.
Note for Subawards or Multi-Project Awards
- Institutions are required to submit copies of contracts specific to senior/key-personnel foreign appointments and/or employment with a foreign institution for all foreign activities and resources that are reported in Other Support. Personal service contracts for lab staff do not need to be provided.
- Translations are required, if they are not in English. Note, this does not include personal service contracts, or employment contracts for fellows supported by foreign entities. NIH will accept machine-read translations, such as Google Translate. Your Central SPO office will translate this for you.
- These contracts MUST be uploaded at time of JIT, following the Other Support documents.


- In-kind contributions, e.g. office/laboratory space, equipment, supplies, employees, students.
- Paid-Direct Graduate Students or Post Docs, performing research activities in the lab is a resource available in support of the PI or other key personnel.This must be reported as in-kind contribution in Other Support. If the relationship is solely mentor/mentee, then this is not a resource.
- If the time commitment or dollar value of the in-kind contribution is not readily ascertainable, the recipient must provide reasonable estimates. Researchers can list zero effort, but must provide the estimated $ value of the in-kind resource. The effort and $ value cannot be both be zero.
| Do in-kind contributions that will be used for the project being proposed need to be included in Other Support? | If an in-kind contribution is listed in Facilities and Other Resources or Equipment, does it also need to be included in both Other Support? | How should researchers list materials (e.g., data, samples, etc.) received from external collaborators on Other Support? | When disclosing materials received from external collaborators on Other Support how far back in time should recipients go, if they are still in use? |
|---|---|---|---|
| If an in-kind contribution, such as technology, chemicals, etc. is intended for use on the project being proposed to NIH in the application, the information must be included as part of the Facilities and Other Resources or Equipment section of the application and does not need to be replicated on Other Support. | If an in-kind contribution is not intended for use on the project being proposed, then the information must be included as part of Other Support. If the in-kind contribution is intended for use on the project being proposed, then information must be included as part of the Facilities and Other Resources or Equipment section of the application and does not need to be replicated on Other Support. | Information on materials received from collaborators must be included in the in-kind contribution section of Other Support, including the source, a summary of the in-kind contribution, and the estimated value. Only resources uniquely available to the researcher must be reported. | Materials provided within the past 3 years, that are still in use, must be included in Other Support. |


- Note, when close to NIH’s fiscal year-end, this timeline may be decreased due to NIH's reduced timeline for these required documents.
For more information or questions email researchadmin@ucsd.edu.