Gene Drives
Mitigate hazards when conducting synthetic gene drive research.
Overview
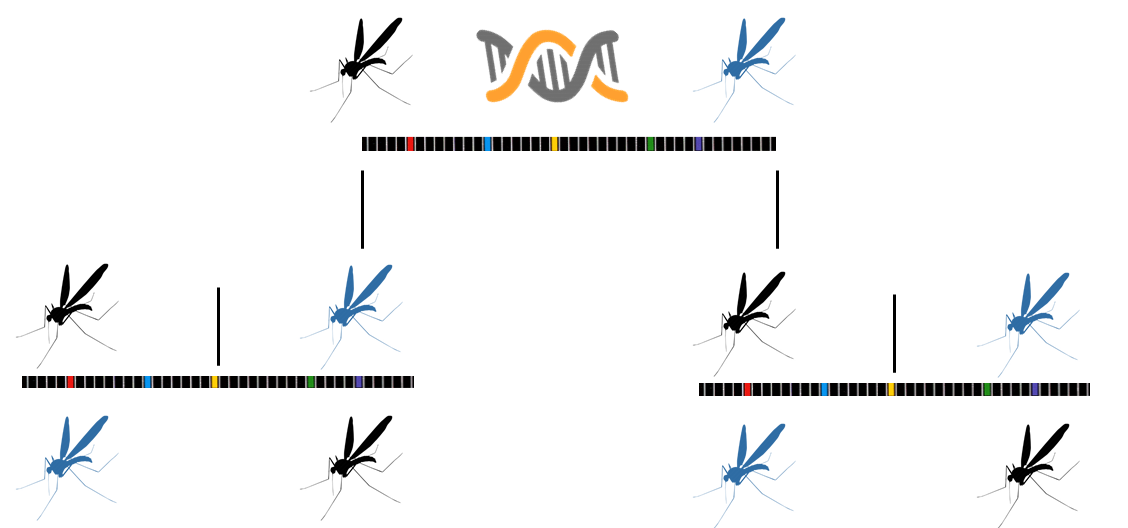
Synthetic gene drives can deliver constructs to an embryo or germ line cell that result in propagation of a gene through the progeny in a greater percentage than Mendelian genetics would predict. The CRISPR/Cas9 based synthetic gene drive consists of three components in one construct: 1) guide RNA containing a gene of interest, 2) the Cas9 gene, and 3) homologous flanking arms that target the desired insertional site. This leads to an autocatalytic insertional mutation in the second allele, resulting in homozygous mutations. This gene drive has been termed mutagenic chain reaction (MCR). Synthetic gene drive research has been a hot topic in the news lately. Tools like CRISPR/Cas9, transcription activator-like effector nucleases (TALEN), and zinc finger nucleases are increasing in popularity and make gene editing quicker and more efficient than other methods.
As gene drive research increases on campus, it’s important to identify the appropriate containment needs for this research in animals and plants. Synthetic gene drive research is currently not approved in humans.
Risks
The primary risk associated with synthetic gene drives in animals is a release of the gene drive into the environment by way of escape or improper disposal of the organism. If the gene drive is used with a lentiviral vector system, accidental inoculation of a researcher could cause the gene drive to be inserted into the human genome with unexpected results. This has led to further examination of the potential risks of gene drive research by the Institutional Biosafety Committee (IBC).
A subcommittee of IBC members and UC San Diego Principal Investigators performing gene drive experiments met in February 2016 to discuss the safety issues surrounding gene drive research. The subcommittee came up with some safety measures which will assist labs in addressing the risks of gene drive research:
- A new Containment Plan for Research with gene drives must be approved by the Institutional Biosafety Committee (IBC) prior to initiation of the proposed experiments. Use the online form to complete a Containment Plan for Research with gene drives.
- The Biohazard Use Authorization (BUA) must specify the “gene drive” category under “nucleic acids” in the list of materials being used
- A short training (to be developed soon) must be completed by researchers working with gene drives
It is important to note that work with CRISPR/Cas9 that does not contain a gene drive, has not shown to be of any greater risk than other well-established gene editing techniques; therefore, it does not require any special containment practices beyond the normal requirements of the designated biosafety level.
If you have questions about gene drive research or any other biosafety concerns, please feel free to contact the UC San Diego biosafety program. Also, a consensus set of guidelines for laboratory confinement of gene drive research published by a consortium of researchers may be helpful.
Resources
- V. Gantz, E. Bier, The Mutagenic Chain Reaction: A Method for Converting Heterozygous to Homozygous Mutations, Science, 348, 442 (2015).
- Akbari, O.S., H.J. Bellen, E. Bier, et al., (+24 other authors) (2015). Safeguarding Gene Drive Experiments in the Laboratory. Science 349, 927-29; Epub 7/30/2015 ScienceExpress DOI: 10.1126/science.aac7932.