Kuali Research (KR)
Information on the Sponsored Research Enterprise System Kuali Research (KR).
Kuali Research (KR) is UC San Diego's campus enterprise system and serves as the official institutional record of proposals, awards, and outgoing subawards. KR integrates and aligns all elements of the research lifecycle including PI certification, detailed budgets, scope of work, compliance reviews, sponsor requirements, and other elements essential for complying with Federal, State, and UC Regulations and Policies.
About the Kuali Research Modules
- Proposal Development (PD): This module manages all aspects of the proposal including a) proposal development, b) campus compliance requirements, c) proposal review and submission, and d) award acceptance stage. PD systematically encompasses compliance, PI certification, detailed budgets, scope of work, sponsor requirements, etc., and is routed for review and submission to the appropriate sponsored project office.
- Institute Proposal (IP): This module manages the final official record of the proposal as submitted to the sponsor, and incorporates all the compliance statuses to ensure these are completed prior to notice of award from the sponsor
- Award: This module manages the complete record of the notice of award from the sponsor, and represents the institution's and sponsor's final commitments for awarded proposals.
- Outgoing Subaward: This module manages the outgoing subawards that transfer a portion of the research and effort of a prime award to another institution. Also known as a flow‐through award.
- Conflict of Interest (COI): This module is a secure, user-friendly, web-based system for UC San Diego faculty and staff to submit and manage their conflict of interest disclosure activities.
- Institutional Review Board (IRB): This module Kuali IRB is the campus administration system for the submission and review of human subject research protocol applications.
How to Request Access
NOTE:
1. In the UC San Diego Support & Services Research Portal, select 'Kuali Research Access Request'
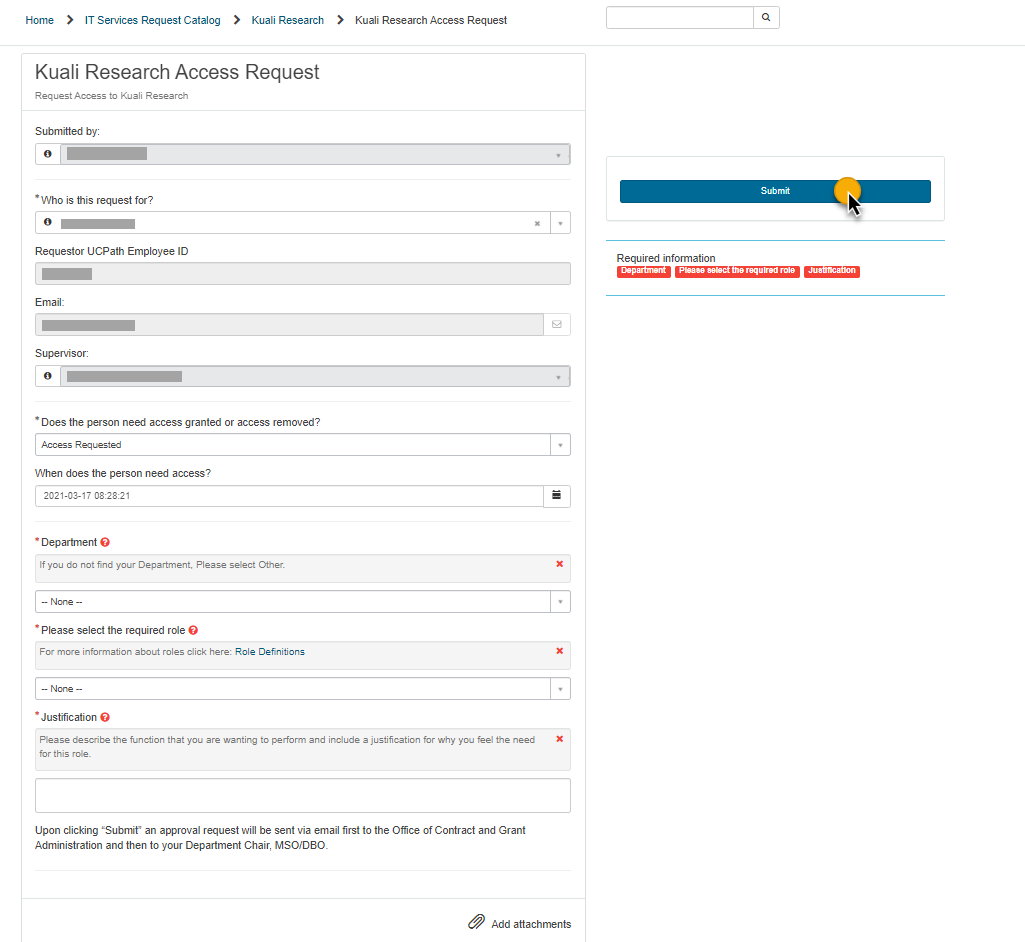
2. Complete the Kuali Research Access Request form and select 'Submit'
System Access Roles and Definitions
NOTE:
- Central Office Award Analyst: Can create Proposal Development (PD) records in any unit. Can view/edit any PD record in any unit. Can create and edit Address Book entries. Can view/edit any Institutional Proposal (IP) record. Can create/edit Negotiations. Can view Awards, Award Attachments, Time & Money Documents, Award Budgets, Negotiations, and Subawards. Can release record locks for any record. Can submit PD records to sponsor. This role is suitable for central office Contract and grant Officers and Compliance Analysts who are responsible for reviewing, approving, submitting, and maintaining research agreements on behalf of UCSD. Can create/edit Awards, Award Attachments, Time and money Documents, Award Budgets, Negotiations, and Subawards. Can bypass required approvers for any PD record that is in workflow routing. Can create/edit Organizations. This role is suitable for central office personnel responsible for creating and maintaining awarded research agreements, obligating funds, posting awards to the financial system, closeouts, accounting, budgeting, and allocations such as the Compliance Analysts, SPO Award Services Team, and SPF Accountants.
- Central Office Proposal Analyst: Can create Proposal Development (PD) records in any unit. Can view/edit any PD record in any unit. Can create and edit Address Book entries. Can view/edit any Institutional Proposal (IP) record. Can create/edit Negotiations. Can view Awards, Award Attachments, Time & Money Documents, Award Budgets, Negotiations, and Subawards. Can release record locks for any record. Can submit PD records to sponsor. This role is suitable for central office Contract and grant Officers and Compliance Analysts who are responsible for reviewing, approving, submitting, and maintaining research agreements on behalf of UCSD. Can bypass required approvers for any PD record that is in workflow routing. May NOT create/edit Awards.12
- Central Office Viewer: Can view any Proposal Development (PD) record in any unit. Can view Institutional Proposal (IP) records in any unit. Can view Awards, Award Attachments, Time & Money Documents, Negotiations, Subawards, and Award Budgets in any unit. May NOT create or edit records. This role is suitable for university administrators responsible for running/maintaining the university and who can be trusted not to disclose sensitive information to unauthorized parties.
- Central Office UCSD Proposal Approver: Person who is responsible for reviewing, approving, and submitting an individual Proposal Development (PD) record to the sponsor. For contracts and other miscellaneous research agreements, this is the person who is responsible for reviewing and approving the proposed research agreement developed and submitted in the PD system.
- Department Academic Approver: Primary departmental approver for Proposal Development (PD) records that require departmental review and approval by the Academic Approver or unit head. The approver can view any PD record that must be approved by the unit head before it can be submitted to the sponsor. This role is suitable for academic unit heads such as Department Chairs, Organized Research Unit Directors, or Deans.
- Department Academic Approver Alternate: Alternate departmental approver for Proposal Development (PD) records that require departmental review and approval by the Academic Approver or unit head. The approver can view any PD record that must be approved by the unit head before it can be submitted to the sponsor. This role is suitable for assistant or associate unit heads such as Vice Chairs, Assistant Organized Research Unit Directors, Associate Organized Research Unit Directors or Associate Deans. A user assigned this role can approve a PD record on behalf of the Academic Approver or unit head.
- Department Administrative Approver: Primary departmental approver for Proposal Development (PD) records that require departmental review and approval by the Academic Approver or unit head. The approver can view any PD record that must be approved by the Academic Approver. This role is suitable for the administrative heads of academic units such as Management Services Officers, Department Business Officers, or Chief Administrative Officers.
- Department Administrative Approver Alternate: Alternate departmental approver for Proposal Development (PD) records that require departmental review and approval by the Academic Approver or unit head. The approver can view any PD record that must be approved by the Academic Approver. This role is suitable for assistant administrative unit heads such as Assistant Management Services Officers, Assistant Department Business Officers, or Assistant Chief Administrative Officers. A user assigned this role can approve a PD record on behalf of the Administrative Approver.
- Department Proposal Viewer: Can view any Proposal Development (PD) records owned by the unit specified in the Group name. Can view Institutional Proposal (IP) records, Awards, Award attachments, Time & Money Documents, and Award Budgets where the lead unit group name is the lead unit of that proposal. May NOT view PD or IP records created by other users in ANY UNIT unless the user is also assigned the PI role on that record. This role is suitable for Unit Heads / Chairs and Administrative Officers who are responsible for running/maintaining this department.
- Department Proposal Creator: Can create Proposal Development (PD) records within the unit specified in the access request. Can ONLY view and edit PD records created by this user. Can submit PD records for review and approval that were created by this user. May NOT view PD or IP records created by other users in ANY UNIT including their own, unless the user is also assigned the PI role on that record. This role is suitable for anyone who needs to create and submit research proposals.
- Department Research Administrator: Can create Proposal Development (PD) records owned by the unit specified in the Group name. Can ONLY view/edit PD records where the lead unit group name is the lead unit of that proposal. Can create and edit Address Book entries. Can view Institutional Proposal (IP), Award, Award attachments, Time & Money Documents, and Award Budgets where the lead unit group name is the lead unit of that proposal. May NOT view PD or IP records created by other users in ANY UNIT unless user is also assigned the PI role on those records. This role is suitable for Research Administrators who support multiple researchers in this department.
Create a Proposal Development (PD) Record
Why Principal Investigator (PI) Certifications are Important
Research at an accredited academic institution is complex and highly regulated. With a recent uptick in sponsors, the U.S. government, and public scrutiny, it has become critical to emphasize full adherence to upholding institutional commitments to support proposed research applications. One of these commitments is to ensure Principal Investigators (PI) respond to the Kuali Research proposal development Compliance Questionnaire and complete the PI Certification.
The Compliance Questionnaire is an important element when preparing each research application because it collects essential project information for which the PI is uniquely qualified to ensure the answers are factually accurate. Incomplete or incorrect answers to the Research Questionnaire pose a significant risk for compliance with institutional and sponsor requirements for conflict of interest, export control, foreign influence, human research, health and safety, and PI eligibility. The answers to the questions trigger systematically designed institutional actions that ensure a) flow of information to appropriate compliance offices, b) conforms with federal, state, and UC requirements and c) accurately classify the project for transparency and institutional reporting.
The PI Certification provides the assurances necessary to acknowledge federal, state and UC required institutional obligations to our sponsors on behalf of the PI. These assurances include:
- True, complete, and accurate information is being submitted
- Acknowledgment that any false, fictitious, or fraudulent statements or claims may subject the PI to criminal, civil, or administrative penalties
- The PI accepts responsibility for the scientific conduct of the project and provides the required progress reports if an award is issued as a result of the application
- Confirmation that neither the PI nor anyone on the research team is currently Debarred, Suspended, or proposed for debarment or Suspension and they will notify the University immediately if this status changes
- No federal funds were used for lobbying activities related to the proposal
For these reasons, it is important that the PI and not an administrator complete the Compliance Questionnaire. While many PIs are already adhering to this expectation, some still allow administrators to complete these sections of the Kuali Research Proposal Development record on their behalf. While this practice may be logistically required under unique and unusual circumstances, the expectation of both the sponsor and UC San Diego is that it is imperative for the PI to complete the Compliance Questionnaire and certification prior to proposal submission.
When are Department Approvals Required
Departmental approval is only required if one or more of the following critical criteria has been identified:
- Cost Sharing
- PI Exception is Required
- New Space
If an agreement does not contain one or more critical criteria, the Kuali Proposal Development (KR PD) record will route directly to the Sponsored Projects Office (SPO).
Training, Resources and Support
Create a Kuali Research Record: For tips and guidance related to entering various agreements into the enterprise system of record.
Kuali Research Systems Training: To register for various eCourses and Virtual Instructor Led training related to the various Kuali modules and to access the Kuali Research Training Guides.
Additional Resources and Help: To get more information on various resources, search the knowledge base and how to contact a Research Administration Client Experience agent.