Dual Use Research of Concern (DURC)
Dual Use Research of Concern is life sciences research that based on current understanding can be reasonably anticipated to provide knowledge, information, products, or technologies that could be misapplied to pose a significant threat, to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.
The United States government created DURC policies to raise awareness and limit potential misuse of scientific information derived from life sciences research.
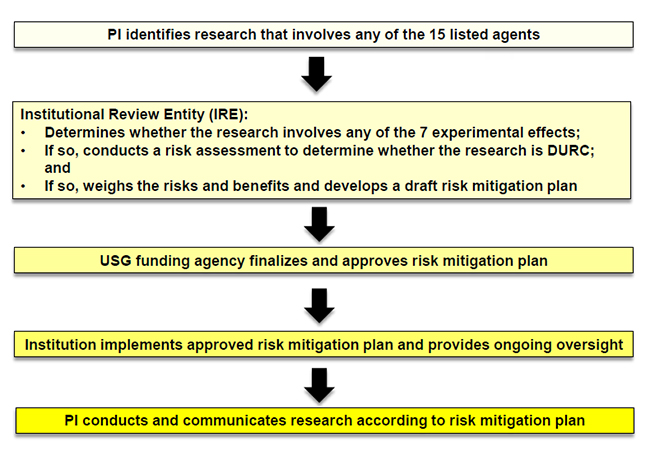
The UCSan Diego Institutional Review Entity (IRE) reviews research that:
- Involves one or more of the fifteen DURC agents and/or toxins (see below “DURC Agents”) currently listed in the US Government DURC Policy; and
- Produce or can reasonably be anticipated to produce, one or more of the 7 experimental effects of concern (see below “DURC Experimental Effects of Concern”) listed in the Policy.
Principal Investigators (PIs) must notify the Institutional Contact for Dual Use Research (ICDUR) that they are using DURC agents through the Biohazard Use Authorization program. See PI Responsibilities.
Review and oversight of DURC are outlined in the University of California policy on DURC.
PI Responsibilities
PIs are responsible for identifying and notifying the IRE when their research may be DURC and implementing a Risk Mitigation Plan when required by IRE.
Assessment
Before commencing research with DURC Agents, the PI must first determine whether the research:
- Directly involves non-attenuated forms of one or more of the potential DURC Agents; or
- Produces or aims to produce or can reasonably be anticipated to produce one or more Experimental Effects of Concern with non-attenuated forms of one or more of the potential DURC Agents; or
- May meet the definition of DURC.
If research does not meet the DURC criteria, the PI must monitor research on an ongoing basis and notify the IRE of changes that may alter the initial IRE determination. PI should consult with Export Control if using a DURC agent and/or toxin.
Submit summary
The PIs will submit documentation indicating reasons research does or does not involve DURC through the existing Biohazard Use Authorization program. The ICDUR will register and retain documentation for review by the UCSD DURC Institutional Review Entity (IRE).
Implement risk mitigation
If research is DURC, the PI is expected to:
- Collaborate with the IRE to develop and implement the Risk Mitigation Plan.
- Notify the ICDUR of any substantive changes in the conduct of the DURC, including if it may no longer be DURC;
- Ensure that laboratory personnel (i.e., those under the supervision of laboratory leadership, including graduate students, postdoctoral fellows, research technicians, laboratory staff and visiting scientists) conducting research with one of more of DURC Agents received appropriate education and training on DURC;
- Be knowledgeable about and comply with all UC and federal policies and requirements for oversight of DURC; and, Communicate about the DURC in a responsible manner and in compliance with the approved risk mitigation plan.
Communicate about the DURC in a responsible manner and in compliance with the approved risk mitigation plan.
DURC Agents
- Avian influenza virus (highly pathogenic)
- Bacillus anthracis
- Botulinum neurotoxin (For purposes of this Policy, there are no exempt quantities of botulinum neurotoxin. Research involving any quantity of botulinum neurotoxin should be evaluated for DURC potential.)
- Burkholderia mallei
- Burkholderia pseudomallei
- Ebola virus
- Foot-and-mouth disease virus
- Francisella tularensis
- Marburg virus
- Reconstructed 1918 Influenza virus
- Rinderpest virus
- Toxin-producing strains of Clostridium botulinum
- Variola major virus
- Variola minor virus
- Yersinia pestis
DURC Experimental Effects of Concern
- Enhances the harmful consequences of the agent or toxin.
- Disrupts immunity or effectiveness of an immunization against the agent or toxin, without clinical and/or agricultural justification.
- Confers to the agent or toxin resistance to clinically and/or agriculturally useful prophylactic or therapeutic interventions or facilitates their ability to evade detection methodologies.
- Increases the stability, transmissibility, or the ability to disseminate the agent or toxin.
- Alters the host range or tropism of the agent or toxin.
- Enhances the susceptibility of a host population to the agent or toxin.
- Generates or reconstitutes an eradicated or extinct agent or toxin listed in the definition of DURC Agents.
UCSD DURC Institutional Review Entity (IRE)
The UCSD DURC IRE evaluates research that involves one or more of the fifteen DURC agents and/or toxins and research which produces, aims to produce, or can be reasonably anticipated to produce one or more of the 7 experimental effects of concern.
IRE is administratively managed and supported by Environmental Health and Safety. For questions related to the IRE, contact DURC-IRE@ucsd.edu.
UCSD Institutional Contact for Dual Use Research (ICDUR)
UC San Diego’s Vice Chancellor for Research designates Laura Provencher, Director of Export Control, to serve as UC San Diego’s internal resource for issues regarding compliance with and implementation of the requirements for oversight of Dual Use Research of Concern (DURC) as well as the liaison between the University and the relevant Federal funding agencies.
For guidance or assistance with DURC related matters, please email Ivan Hernandez, Export Analyst and DURC work lead at i6hernandez@ucsd.edu or Laura Provencher at lprovencher@ucsd.edu.
Procedure for DURC Research
Initial review
If the IRE determines the research meets the definition of DURC, it will promptly notify the PI and the applicable US funding agency within 30 calendar days. The IRE will work with the PI to create a Risk Mitigation Plan. This process is outlined in detail in the UC DURC policy. Once the U.S. funding agency approves of the draft Plan, the IRE will present the final Risk Mitigation Plan to the PI. The ICDUR will collaborate implementation with the PI.
Continuing research
The IRE will review at least annually all active Risk Mitigation Plans at the University. The IRE and the PI will modify Risk Mitigation Plans as needed to mitigate the DURC risks.
Export Controls and DURC
The fifteen agents listed in the DURC oversight policies are all regulated by the Export Administration Regulations (EAR) in Category XIV of the International Traffic in Arms Regulations (ITAR) may apply to DURC items. The export of DURC agents requires a license. Export licenses may also be necessary for foreign person access to confidential, proprietary or information. Licensing takes a minimum of 6 weeks and must be in place prior to export.
When applicable, Export Control will advise researchers on any export licensing obligations, required control plans, and file export license applications.
DURC and Subawards
Federal DURC policy requires that the institution receiving U.S. federal funding notify the U.S. funding agency sponsor when elements of a potential DURC project are sub awarded to other institutions. The grantee (prime awardee/institution) must provide copies of each sub awardee institution’s Risk Mitigation Plan. UCSD will work with the prime institution to confirm that DURC oversight is consistently applied by all participating entities under the award. If the prime institution’s procedures or standards are less rigorous than the sub awardee’s, the more rigorous standard will be applied.
Additional Resources
- Federal Government DURC Policy & Guidance
- United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern (March 2012, PDF)
- United States Government Policy for Institutional Oversight of Dual Use Research of Concern (September 2014, PDF)
- FAQ on USG Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern (PDF)
- National Institutes of Health – Office of Science Policy: Dual Use Research of Concern
- Tools for the Identification, Assessment, Management, and Responsible Communication of Dual Use Research of Concern: A Companion Guide to the United States Government Policies for Oversight of Life Sciences Dual Use Research of Concern (PDF)
- Training Slide Set: Training on the USG Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern (PDF)